It is a truth universally acknowledged among virologists that a single virus, carrying a full set of genes, must be in want of a cell. A virus is just a collection of genes packaged into a capsule. It infiltrates and hijacks a living cell to make extra copies of itself. Those daughter viruses then bust out of their ailing host, and each finds a new cell to infect. Rinse, and repeat. This is how all viruses, from Ebola to influenza, are meant to work.
But Stéphane Blanc and his colleagues at the University of Montpellier have shown that one virus breaks all the rules.
Faba bean necrotic stunt virus, or FBNSV for short, infects legumes, and is spread through the bites of aphids. Its genes are split among eight segments, each of which is packaged into its own capsule. And, as Blanc’s team has now shown, these eight segments can reproduce themselves, even if they infect different cells. FBNSV needs all of its components, but it doesn’t need them in the same place. Indeed, this virus never seems to fully come together. It is always distributed, its existence spread between capsules and split among different host cells.
“This is truly a revolutionary result in virology,” says Siobain Duffy of Rutgers University, who wasn’t involved in the study. “Once again, viruses prove that they’ve had the evolutionary time to try just about every reproductive strategy, even ones that are hard for scientists to imagine.”
Read: How viruses cooperate to defeat CRISPR
FBNSV is one of several “multipartite viruses” that split their genes among different capsules. These oddballs were first discovered in the 1940s, and though they account for about 20 percent of known viral species, they’re still rather obscure. Blanc thinks that’s because they almost always infect plants and fungi, and only two have been found in animals—one in a moth and one in a mosquito. “I lecture on several virology courses, and even people in Ph.D. programs haven’t heard of them,” he laments. “They’re everywhere, but because they’re mainly on plants, no one cares.”
These viruses have always been baffling, even to virologists who knew about them. Everyone assumed that they could only reproduce if all the segments infected the same host cell. But the risk of losing a piece, and so dooming the others, skyrockets as the number of pieces goes up. In 2012, two researchers calculated that the odds of successfully getting every segment in the same cell become too low with anything more than three or four segments. FBNSV, with its eight segments, “should never have evolved,” Blanc says. Its mere existence suggests “that something must be wrong in the conceptual framework of virology.”
Perhaps, he realized, these viruses don’t actually need to unite their segments in the same host cell. “If theory was saying that this is impossible, maybe the viruses just don’t do it,” he says. “And once we had this stupid idea, testing it was very easy.”
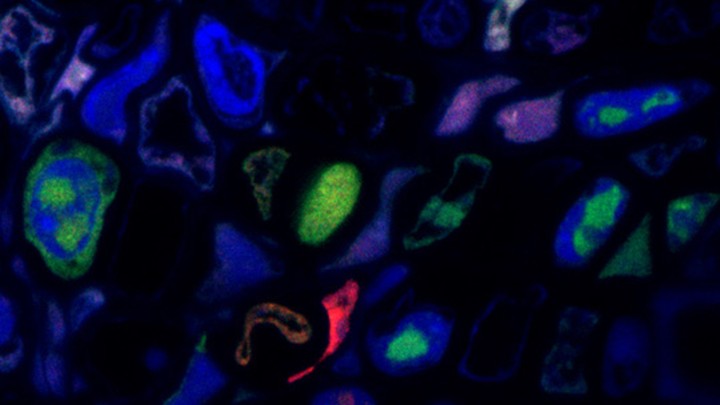
His colleagues Anne Sicard and Elodie Pirolles labeled pairs of FBNSV’s genes with molecules that glowed in different colors—red for one segment, for example, and green for another. Then, they simply looked down a microscope to see whether the colors overlapped in the same cells. They almost never did. When the team first saw that, “we were jumping and running around the lab,” Blanc says. “But we were also scared about it being a [mistake]. We took six years to verify it.”
For example, they showed that the levels of one segment aren’t tied to the levels of another, as you would expect if they were replicating in the same host cell. Instead, in any one infected plant, the different segments seem to accumulate at different rates.
But that discovery raised another problem. Each of the eight segments carries a gene with its own vital role. One makes the proteins that copy the virus’s DNA once it gets inside its hosts. Another creates the proteins that form the virus’s capsules. See the problem? If these segments end up in different cells, the DNA-copying one shouldn’t be able to make capsules, the capsule-making gene shouldn’t be able to copy itself, and both of them would be stuck.
That doesn’t happen, the team discovered, because the virus’s genes might be stuck in neighboring cells, but the proteins created by those genes can move. The capsule-making protein can get into a cell with the DNA-copying gene, and cover it. The DNA-making protein can get into a cell with the capsule-making gene, and copy it. Think of the eight segments as factories in different cities, shipping assembly robots to one another so that each site can manufacture its own separate product. It is within this expansive trade network that the distributed virus truly exists.
Read: The oldest virus ever sequenced comes from a 7,000-year-old tooth
It’s not clear how this network operates, but many scientists have found that plant proteins can voyage between cells, even over long distances from root to shoot. Some researchers who study multipartite viruses have even suggestedthat they could make use of these botanical highways. But Blanc’s team has now found clear and unambiguous evidence that they do. Perhaps, he says, “this is why multipartite viruses don’t exist so much in animals. Maybe it’s harder for our proteins to travel between cells.”
“The work is very important … and very carefully done,” says Marilyn Roossinck of Pennsylvania State University. For decades, she has been studying a different multipartite virus that affects cucumbers, and though she has seen some of the patterns that Blanc’s team did, “these were never published, as their significance wasn’t clear,” she says.
“This report challenges a fundamental assumption of virology,” adds Rodrigo Almeida of the University of California at Berkeley, who studies plant diseases. “I am not aware of any similar example in biology, where genetic information appears to be split among host cells.”
The closest example I can think of exists in cicadas. These noisy insects rely on a bacterium called Hodgkinia, which lives inside their cells and provides them with nutrients. But this one bacterium has fractured into several daughter species, each of which contains just a few of Hodgkinia’s full set of genes. None of these partial microbes can survive on its own; they only function as a set. But these daughter species are all still locked within the same cell, so they’re not truly distributed as the virus is. They are also problematic: If any of them were to disappear, the rest would also die out, as would their cicada host. Hodgkinia’s fragmented existence is a looming disaster—“a slow-motion extinction event,” according to John McCutcheon, who described it.
By contrast, multipartite viruses are clearly very successful, so their bizarre distributed existence must have some benefit. And Blanc thinks he knows what that might be.
His team has shown that when FBNSV infects a plant, the frequency of each segment is very predictable. Some of them are common and others are rare, but their relative proportions are constant, at least within a given species of plant. If the virus infects a different plant species, those proportions change—to a different, but still predictable, pattern. Blanc calls these “genome formulas”—ratios of genes that FBNSV uses for different hosts.
The virus’s use of these formulas reminds Blanc of the ways in which animals and other complex organisms adapt to different environments by tweaking the numbers of important genes. In very rough terms, the more copies you have, the more effectively that gene can do its thing. But viruses are tiny entities, whose capsules only have room for small genomes. There’s not enough space for them to just wantonly double their gene counts.
Multipartite viruses don’t have to. If they want to emphasize the use of a certain gene, they just need to get the segment carrying it into more host cells. “This lifestyle allows the virus to adjust its gene copy number without mutating,” Blanc says. It’s as if FBNSV has found a way to have the flexibility of a much larger and more complex genome, while still keeping the unflinching efficiency of a virus.
Read: The viruses that eavesdrop on their hosts
These discoveries could also change our understanding of other more traditional viruses. Influenza’s genome is split into eight segments, and unlike FBNSV, all of these are packaged into the same capsule. Researchers typically assume that every capsule contains the full octet, but in 2013, Christopher Brooke of the University of Illinois showed that 90 percent of them are missing at least one segment. Influenza virus “exists primarily as a swarm of complementation-dependent, semi-infectious virions,” Brooke wrote.
Three years later, a different team showed that the same is true for the virus behind Rift Valley fever: Only a minority contain all three of the virus’s gene segments, and most are missing one. “Perhaps the boundary between these viruses and the multipartite ones isn’t so clear,” Blanc says.
Many viruses also produce capsules called “defective interfering particles,” which … well, the clue’s in the name. They’re defective because, for some reason, they’ve lost part of their full genome. They’re interfering because, though they’re defective, their parent viruses will still make copies of them, flooding the total pool of capsules with noninfective deadbeats. “These things have been known for a century, and they’ve long been considered as junk,” Blanc says. “But they are very efficiently maintained in any viral infection. Maybe they can profit from the system we have identified.”
 1 New Concepts in Science*
1 New Concepts in Science*