SPL
Solutions of quantum dots made from cadmium selenide respond to ultraviolet light by emitting visible light in specific wavelengths.
At Biopolis, a sprawling research complex in Singapore, Chi Ching Goh leans over an anaesthetized mouse lying on the table in front of her, and carefully injects it with a bright yellow solution. She then gently positions the mouse's ear underneath a microscope, and flips a switch to bathe the ear in ultraviolet light. Seen through the microscope's eyepiece, the illumination makes the blood underneath the skin glow green, tracing the delicate vessels that carry the solution through the creature's body.
Ultimately, Goh, a PhD candidate at the National University of Singapore, hopes that the method will help her to find blood vessels that are leaking owing to inflammation, perhaps helping to detect malaria or predict strokes. Crucial to her technique are the virus-sized particles that give the solution its colour. Just a few tens of nanometres across, they are among a growing array of 'nanolights' that researchers are tailoring to specific types of fluorescence: the ability to absorb light at one wavelength and re-emit it at another.
Many naturally occurring compounds can do this, from jellyfish proteins to some rare-earth compounds. But nanolights tend to be much more stable, versatile and easier to prepare — which makes them attractive for users in both industry and academia.
The best-established examples are quantum dots: tiny flecks of semiconductor that are prized for their beautiful, crisp colours. Now, however, other types of nanolight are on the rise. Some have a rare ability to absorb lots of low-energy photons and combine the energy into a handful of high-energy photons — a trick that opens up opportunities such as producing multiple colours at once. Others are made from polymers or small organic molecules. These are less toxic than quantum dots and often outshine them — much to the amazement of chemists, who are used to carbon-based compounds simply degrading in the presence of ultraviolet light.
“I was kind of surprised to find that we can make organic particles much brighter than inorganic particles,” says Bin Liu, a chemical engineer at the National University of Singapore and the designer of the fluorescent nanoparticles that Goh is using.
Nanolights have already begun to find application in areas ranging fromflat-screen displays to biochemical tests. And researchers are working towards even more ambitious uses in fields such as solar energy, DNA mapping, motion sensing and even surgery. “The research is certainly fast-paced,” says Daniel Chiu, who studies fluorescent nanoparticles at the University of Washington in Seattle.
It is also increasingly wide ranging, adds Paul Alivisatos, a chemist at the University of California, Berkeley, and a co-founder of the first quantum-dot technology companies. “It's so much fun now.”
Size matters
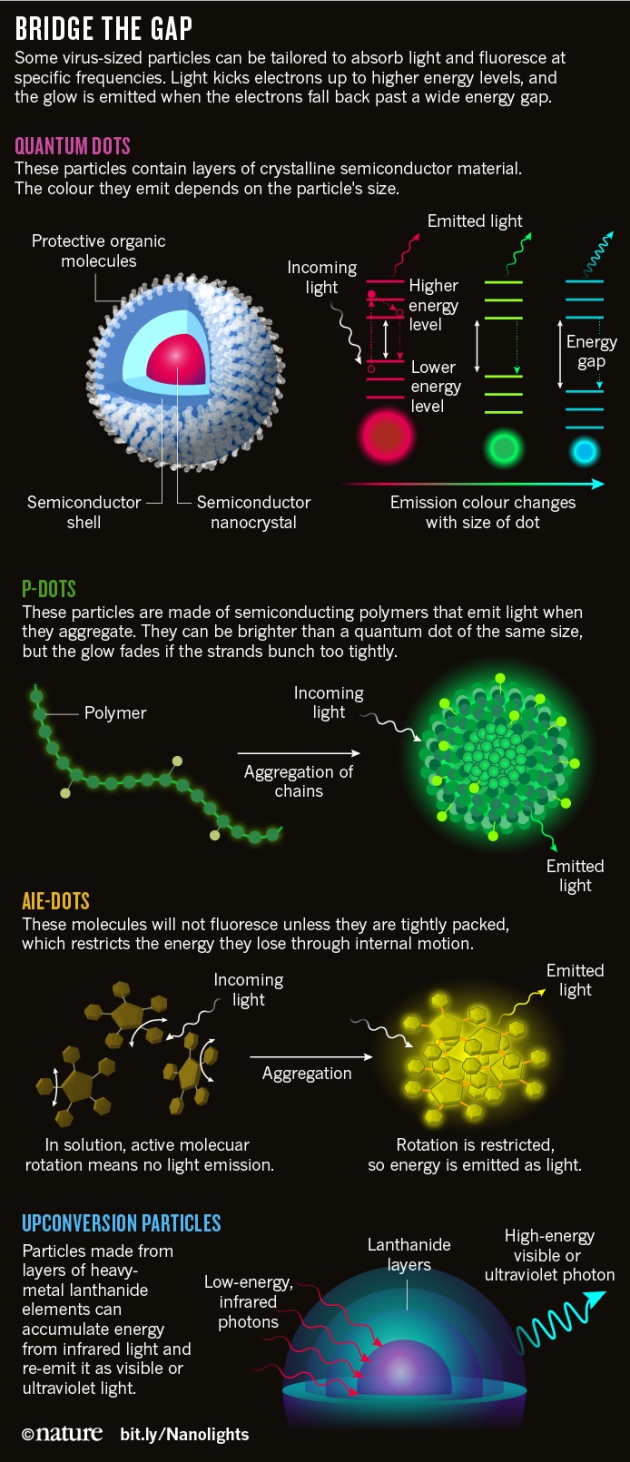
The nanolight era began with the discovery of quantum dots in 1981. Russian physicists were growing tiny crystals of the semiconductor cuprous chloride in silicate glass and observed that the colour of the glass depended on the size of the particles1. The crystals were so small that quantum effects were kicking in and they were behaving somewhat like atoms: they could absorb or emit light only as specific colours, with the exact frequencies depending on the size or shape of the particles (see 'Bridge the gap').
Nik Spencer/Nature
The quantum dots were bright and beautiful, says Yin Thai Chan, who studies them at the National University of Singapore, but “there were no obvious applications”. By the early 2000s, however, the pure colours had begun to attract television manufacturers, as well as biomedical researchers, who saw their potential for labelling specific proteins and DNA segments.
“Everything is good about quantum dots,” says Liu — except for one thing: their toxicity. The best-performing dots contain cadmium, which can poison cells. This limits their usefulness in biology and in applications such as household electronics, because some countries do not allow use of the element in such devices. To some extent, this problem can be overcome by replacing cadmium with zinc or indium, which are considerably less toxic, or by wrapping cadmium-based quantum dots in polymers that are biocompatible. But the toxicity is still a drawback for researchers who are pursuing ambitious applications such as fluoresence-guided surgery, in which nanoparticles are injected into a tumour, for instance, to make it glow and help surgeons to remove all traces of it.
Going organic
Partly in response to this challenge, researchers have begun to develop nanoparticles from materials that fluoresce naturally. Because the light-emitting properties of these nanolights come from their composition rather than their size or shape, they are easier to make with specific colours. “Practically, this is useful because of the difficulties to synthesize everything in the same size,” says Chiu.
It also frees up nanolight researchers to explore alternative materials, such as semiconducting polymers. Studied for their potential in electronics since the 1950s, these polymers consist of simple compounds linked into a long chain in which electrons are free to move, but only at certain energies determined by the chain's composition.
Light is emitted when electrons are kicked up to higher energy levels by some outside source, such as ultraviolet light, then fall back down to lower levels. The polymers can also be decorated with side groups to give them specific properties — for example, targeting them to cancer cells, or helping them to dissolve in water. And when chains are aggregated into polymer nanoparticles, or 'P-dots', they can be as much as 30 times brighter than a quantum dot of comparable size2.
Semiconducting polymers do tend to be less stable than the inorganic semiconductors used in quantum dots. But because they are based on carbon, and contain no metals, they are much more likely to be biocompatible. P-dots have been used to stain and image cells, and also as sensors to detect oxygen, enzymes or metal ions such as copper.
In 2013, for example, Chiu and his collaborators reported that a P-dot bound to a terbium ion can detect biomolecules produced by bacterial spores3. Under an ultraviolet lamp, the P-dots glow dark blue and the terbium ions emit a faint neon green colour. But when passing biomolecules attach themselves to terbium, the ions' light strengthens to a bright green. The P-dots' light remains unchanged, so it serves as an internal standard.
Unfortunately, P-dots also have a fundamental problem: the polymer molecules are packed together so closely that they can be affected by 'quenching' — a phenomenon in which most of the energy coming from the original light source is quickly dissipated and fails to trigger fluorescence.
Quenching has a huge impact on efficiency, says Yang-Hsiang Chan, a chemist at National Sun Yat-Sen University in Kaohsiung, Taiwan. One way to tackle it is to add bulky groups onto the polymer backbone to prevent the polymers from getting too close to each other. But this can be self-defeating: the resulting nanoparticles tend to be too fat to get into cells, say, or too dim to be useful. “It is very hard to get the right balance,” says Chan, who is working to solve the problem by designing new polymers.
Together we shine
A more fundamental solution was pioneered in 2001, when Ben Zhong Tang at the Hong Kong University of Science and Technology in Clear Water Bay found that a class of small organic molecules would fluoresce only when they aggregate together4. These molecules are shaped like propellers or pinwheels, and they fluoresce when packed because they can no longer move and waste their energy. Instead, they release their energy as light — a phenomenon Tang has named aggregation-induced emission (AIE). He called the molecules AIE-gens.
Over the next few years, Tang and his students changed the side groups and introduced elements such as nitrogen or oxygen, and AIE-gens can now glow in the entire spectrum of colours from ultraviolet to near-infrared. “My students quickly made a lot,” says Tang. “We can change the colour at will.”
“We can change the colour at will.”
In 2011, Tang met Liu through a collaboration at the Institute of Materials Research and Engineering in Singapore, part of the government-backed Agency for Science, Technology and Research (A∗STAR). At that time, AIE-gens were performing well, except that they could not dissolve in water, which made them difficult to use in biological applications. Liu was an expert in making things water-soluble, so Tang gave her some of his best AIE-gens to work with.
Liu solved the problem by experimenting with polymers that are oil-loving on one end and water-loving on the other. The AIE-gens crowd within the polymer's oil-loving ends, and its water-loving ends point outwards to form a protective shell, resulting in a water-soluble capsule with a dense core full of AIE-gens. Liu designed a protective shell for the resulting nanoparticles, called AIE-dots, such that it could be decorated with various chemical groups that are tailored to specific applications. The shell can easily accommodate a wide variety of AIE-gens, says Liu, “so that we can screen a lot of molecules very quickly to find out which one is the best.”
AIE-dots have been used to stain various tissues, from blood vessels to cancer cells to intracellular organelles such as mitochondria. Last year, Liu, Tang and their colleagues reported an AIE-dot that could be useful in a type of light-activated treatment known as photodynamic therapy5. It carries two molecules on its surface: one to get the dot into a cancer cell, and another to make it stick to the mitochondria. Once excited by an external light source, the AIE-dot produces red light that generates oxygen radicals near the mitochondria and kills the cancer cells.
The best AIE-dots can be 40 times brighter than quantum dots6. “With AIE, high density in constrained space produces high brightness,” says Guangxue Feng, a research assistant in Liu's lab. That is particularly useful for applications such as visualization of tissues or long-term tracking of cancer cells, which halve the number of nanoparticles per cell every time they divide.
But the brightness comes at a cost: AIE-dots produce a much broader, more-muted spectrum than the pure, brilliant colours of quantum dots. But that hasn't kept Liu from starting LuminiCell, a spin-off company in Singapore that produces AIE-dots in three colours and three sizes for research such as Goh's at A∗STAR. Tang is also trying to start a company; both he and Liu are now hoping to gain approval from the US Food and Drug Administration to test AIE-dots for human use in applications such as fluorescence-guided surgery.
Into the infrared
Another thing that limits the biological use of nanolights is that most of them absorb ultraviolet or visible light, which can penetrate only a few millimetres into tissue. Longer-wavelength near-infrared radiation can penetrate up to three centimetres — a much better depth for uses such as releasing drugs. But infrared light does not have enough energy to break the bonds that hold drugs on the nanoparticle, so many researchers are turning to a process called upconversion. This involves making material that can absorb multiple low-energy infrared photons, accumulate the energy and then re-emit it as higher-energy ultraviolet or visible photons.
The group of heavy-metal elements known as lanthanides are particularly good at this trick. In 2011, Xiaogang Liu at the National University of Singapore reported that his laboratory had created a particularly versatile type of nanoparticle7 with a Russian doll-like structure. It consists of a series of concentric shells that each contains a different combination of lanthanides. The energy from infrared light is absorbed by the core, then migrates outwards layer by layer, snowballing from lanthanide to lanthanide before finally emerging as high-energy light near the surface.
The 15 lanthanides can be combined in numerous different ways to produce nanoparticles that emit in all colours, sometimes even several at once. In one demonstration, a student in Liu's lab shone an infrared laser through a series of beakers containing clear solutions of the nanoparticles: glowing lines of purple and green light appeared in the beakers where the infrared beam passed through.
Liu thinks that these upconversion nanoparticles have tremendous potential in photovoltaics, where they could help to capture near-infrared light, which makes up almost half of the Sun's radiation. This is a long way from being practical, however: the brightest available nanoparticles convert just 10% of the light they absorb. Liu's group is working to build a library of these nanoparticles — no small task considering the number of lanthanides — to systematically study their properties and work on making them brighter.
Last December, Marta Cerruti, a biomaterials scientist at McGill University in Montreal, Canada, reported a proof-of-concept system in which a lanthanide-containing nanoparticle is coated with a gel that contains a 'drug' — for testing purposes, a compact, stable protein8. After absorbing near-infrared light, the nanoparticle emits infrared, visible and ultraviolet light simultaneously. The infrared emission allows the researchers to track the nanoparticle's location, and the ultraviolet light cleaves the protein's bond to the gel and releases it — or at least, it has in the laboratory. Cerruti's group is now planning tests in animals.
At the end of the day, quantum dots are still the nanolights to beat. “They are the de facto standard,” says Chan. “A lot of the fundamental phenomena concerning light emission are established in quantum dots and it shapes the way others explain what they see.”
Quantum dots are also still a research frontier. For example, they are getting a boost from relatively new semiconducting materials such as the perovskites. Unlike conventional semiconductors, which have a fixed ratio of elements, perovskites can have variable ratios, so researchers can tailor the dots' emission by varying their composition as well as their size. “They have two degrees of freedom for tunability,” says Edward Sargent, a materials engineer at the University of Toronto, Canada.
Last year, Sargent reported a hybrid material in which quantum dots are held within a perovskite9, yielding the kind of high brightness and good electron mobility that manufacturers might like for use in flat-screen displays.
Other researchers are hoping to combine the best properties of each component by pursuing hybrid nanolights. Bin Liu, for example, is trying to blend AIE-dots with quantum dots to produce narrow emissions. And semiconducting polymers paired with AIE-dots can produce much brighter particles than either alone10.
Another grand challenge for nanolights is to create versions that emit infrared wavelengths efficiently. That would open up applications in motion sensing, from tiny detectors that tell the screen to turn off when a mobile phone is lifted to the ear to sophisticated devices for self-driving cars and home monitoring for elderly people. “There's so much more we could do,” says Sargent.
- Nature
- 531,
- 26–28
- ()
- doi:10.1038/531026a
 4 New Concepts in Technologies*
4 New Concepts in Technologies*