In a groundbreaking study published today in Nature, Australian scientists have resolved a long-standing problem in regenerative medicine. Led by Professor Ryan Lister from the Harry Perkins Institute of Medical Research and The University of Western Australia and Professor Jose M Polo from Monash University and the University of Adelaide, the team developed a new method to reprogram human cells to better mimic embryonic stem cells, with significant implications for biomedical and therapeutic uses.
“We predict that TNT reprogramming will establish a new benchmark for cell therapies and biomedical research, and substantially advance their progress.”
Professor Ryan Lister, UWA Centre for Medical Research
In a revolutionary advance in the mid-2000s, it was discovered that the non-reproductive adult cells of the body, called ‘somatic’ cells, could be artificially reprogrammed into a state that resembles embryonic stem (ES) cells which have the capacity to then generate any cell of the body.
The ability to artificially reprogram human somatic cells, such as skin cells, into these so-called induced pluripotent stem (iPS) cells provided a way to make an essentially unlimited supply of ES-like cells, with widespread applications in disease modelling, drug screening and cell-based therapies.
“However, a persistent problem with the conventional reprograming process is that iPS cells can retain an epigenetic memory of their original somatic state, as well as other epigenetic abnormalities,” Professor Lister said. “This can create functional differences between the iPS cells and the ES cells they’re supposed to imitate, and specialised cells subsequently derived from them, which limits their use,”.
Professor Jose Polo, who is also with the Monash Biomedicine Discovery Institute, explained that they have now developed a new method, called transient-naive-treatment (TNT) reprogramming, that mimics the reset of a cell’s epigenome that happens in very early embryonic development.
“This significantly reduces the differences between iPS cells and ES cells and maximises the effectiveness of how human iPS cells can be applied,” he said.
Dr Sam Buckberry, a computational scientist from UWA, the Harry Perkins Institute, and Telethon Kids Institute, and co-first author of the study, said by studying how the somatic cell epigenome changed throughout the reprogramming process, they pinpointed when epigenetic aberrations emerged, and introduced a new epigenome reset step to avoid them and erase the memory.
Dr Xiaodong Liu, a stem cell scientist who also spearheaded the research said the new human TNT-iPS cells much more closely resembled human ES cells – both molecularly and functionally – than those produced using conventional reprograming.
Dr Daniel Poppe, a cell biologist from UWA, the Harry Perkins Institute and co-first author, said the iPS cells generated using the TNT method differentiated into many other cells, such as neuron progenitors, better than the iPS cells generated with the standard method.
Monash University student and co-first author Jia Tan, said the team’s TNT method was dynamite.
“It solves problems associated with conventionally generated iPS cells that if not addressed could have severely detrimental consequences for cell therapies in the long run,” he said.
Professor Polo said the precise molecular mechanisms underlying the iPS epigenome aberrations and their correction were not fully known, and further research was needed to understand them.
“We predict that TNT reprogramming will establish a new benchmark for cell therapies and biomedical research, and substantially advance their progress,” Professor Lister said.
The collaborative research project also included researchers from the Australian National University, Westlake University, Queen Mary University of London, Mater Research Institute, University of Queensland, Queensland Brain Institute, South Australian Health & Medical Research Institute, Duke-NUS Medical School and CSIRO.
Read the full paper ‘Transient naive reprogramming corrects hiPS cells functionally and epigenetically’ in Nature.
Image above title courtesy of Ella Marushchenko
Media references
Doug MacLaurin (UWA Media & PR Advisor) 6488 2802
======
Transient naive reprogramming corrects hiPS cells functionally and epigenetically
Nature volume 620, pages863–872 (2023)
Abstract
Cells undergo a major epigenome reconfiguration when reprogrammed to human induced pluripotent stem cells (hiPS cells). However, the epigenomes of hiPS cells and human embryonic stem (hES) cells differ significantly, which affects hiPS cell function1,2,3,4,5,6,7,8. These differences include epigenetic memory and aberrations that emerge during reprogramming, for which the mechanisms remain unknown. Here we characterized the persistence and emergence of these epigenetic differences by performing genome-wide DNA methylation profiling throughout primed and naive reprogramming of human somatic cells to hiPS cells. We found that reprogramming-induced epigenetic aberrations emerge midway through primed reprogramming, whereas DNA demethylation begins early in naive reprogramming. Using this knowledge, we developed a transient-naive-treatment (TNT) reprogramming strategy that emulates the embryonic epigenetic reset. We show that the epigenetic memory in hiPS cells is concentrated in cell of origin-dependent repressive chromatin marked by H3K9me3, lamin-B1 and aberrant CpH methylation. TNT reprogramming reconfigures these domains to a hES cell-like state and does not disrupt genomic imprinting. Using an isogenic system, we demonstrate that TNT reprogramming can correct the transposable element overexpression and differential gene expression seen in conventional hiPS cells, and that TNT-reprogrammed hiPS and hES cells show similar differentiation efficiencies. Moreover, TNT reprogramming enhances the differentiation of hiPS cells derived from multiple cell types. Thus, TNT reprogramming corrects epigenetic memory and aberrations, producing hiPS cells that are molecularly and functionally more similar to hES cells than conventional hiPS cells. We foresee TNT reprogramming becoming a new standard for biomedical and therapeutic applications and providing a novel system for studying epigenetic memory.
Main
Somatic cell reprogramming requires substantial epigenome remodelling to establish states resembling hES cells. The generation of hiPS cells by the ectopic expression of the transcription factors OCT4, KLF4, SOX2 and MYC (hereafter referred to collectively as OKSM) is the most widely used method9. Despite the high similarity of induced pluripotent stem (iPS) cells and embryonic stem (ES) cells10,11, substantial evidence indicates that iPS cells are epigenetically and functionally distinct from ES cells, including residual somatic cell epigenetic memory and de novo epigenetic aberrations1,2,3,4,5,6,7,8. Previous reports have shown that DNA methylation and histone modifications encode these epigenetic differences, which are transmissible through differentiation1,2,3,4, limiting the potential use of hiPS cells in disease modelling, drug screening and cell therapies12. However, the mechanisms underpinning how aberrant epigenetic states emerge during reprogramming remain unknown.
The observation that cells reprogrammed by somatic cell nuclear transfer (SCNT) retain less epigenetic memory than OKSM-reprogrammed cells13 indicates that epigenetic aberrations are not inherent to reprogramming and can be mitigated. Although the exact mechanisms are unknown, SCNT reprogramming appears to recapitulate the pre-implantation epigenome reset, mediated by the molecular environment within oocytes. Notably, although SCNT stem cells contain less epigenetic memory than hiPS cells13, SCNT reprogramming requires donor oocytes, rendering the method inefficient, complex and unscalable.
Conventional OKSM reprogramming produces hiPS cells in a primed pluripotent state (primed-hiPS cells) resembling post-implantation epiblast cells14,15. Recent developments enable the reprogramming of somatic cells to a naive pluripotent state (naive-hiPS cells) resembling the pre-implantation epiblast, including low global DNA methylation16,17,18. These two reprogramming paradigms provide tractable model systems to study how epigenome resetting is influenced by environments resembling distinct developmental states of pluripotency. Previous studies have focused on changes in DNA methylation when hES cells are switched between primed and naive culture conditions19,20,21, but it is not known whether epigenetic memory and aberrations occur in naive-hiPS cell reprogramming. We therefore set out to study the origins, dynamics and mechanisms of epigenetic abnormalities in naive and primed reprogramming to comprehensively understand the reprogramming process.
Divergent epigenome remodelling in hiPS cells
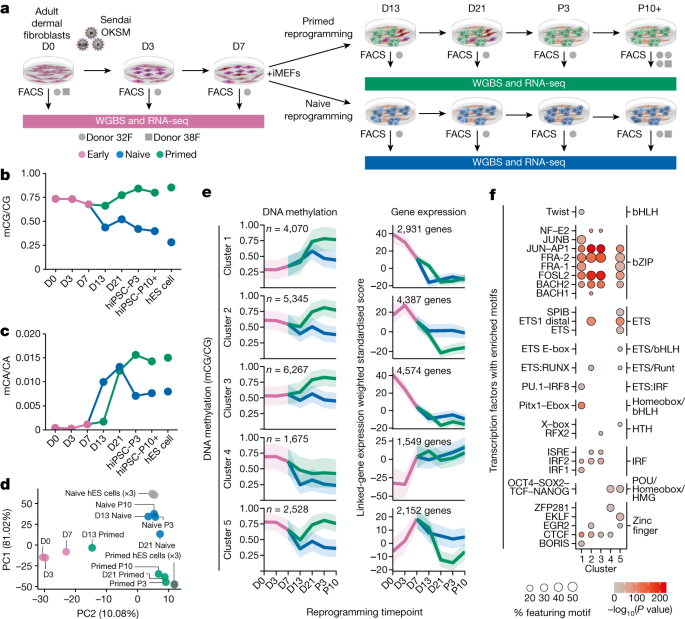
To investigate epigenome remodelling throughout naive and primed reprogramming, we reprogrammed human fibroblasts into both primed and naive pluripotent states using Sendai viral OKSM transcription factors16, and isolated reprogramming intermediates throughout this process using intermediate cell surface markers22 (Fig. 1a, Extended Data Fig. 1a,b and Supplementary Table 1). We then profiled DNA methylation using whole-genome bisulfite sequencing (WGBS) and analysed gene expression data previously generated by RNA sequencing (RNA-seq) from the same cells22 (Fig. 1a). This enabled base-resolution quantification of the methylome throughout reprogramming. The largest changes in CG DNA methylation during primed reprogramming occur between days 13 and 21, with global levels reaching those similar to hES cells by passage 3 (Fig. 1b and Extended Data Fig. 1c). By contrast, most CG methylation changes in naive reprogramming occur before day 13 (Fig. 1b). As expected, naive conditions result in partial methylation at most CG dinucleotides (Extended Data Fig. 1c). Furthermore, intermediate levels of CG methylation in naive conditions is a result of sparse distribution of methylated CGs on individual DNA fragments, demonstrating that intermediate methylation is not caused by cell heterogeneity (Extended Data Fig. 1d).
a, Experimental design for time-course profiling of epigenomic changes that occur as cells are reprogrammed from fibroblasts to naive-hiPS and primed-hiPS cells. iMEFs, irradiated mouse embryonic fibroblasts; FACS, fluorescence-activated cell sorting. D indicates day of experiment and P indicates passage number. b,c, Dynamics of global CG methylation (b) and CA methylation (c) during naive and primed reprogramming compared with primed and naive hES cells. DNA methylation levels were calculated as a coverage-weighted mean (Methods). d, Principal component analysis of CG DNA methylation levels at GeneHancer regulatory elements throughout reprogramming. e, c-Means fuzzy cluster analysis of CG DNA methylation levels in regulatory elements throughout primed and naive reprogramming. Gene-expression plots of genes identified through GeneHancer’s double-elite set of gene–enhancer validated pairs47. The line is the nonparametric bootstrap mean and the ribbon shows the 99% confidence interval. f, Transcription factors (grouped by family) with significantly enriched motifs for DNA binding domains in regulatory elements for each cluster in e. Homer hypergeometric enrichment test; false discovery rate (FDR) < 0.01.
CpH methylation (where H represents A, C or T) is a hallmark of pluripotent stem cells, and is mostly attributable to CA methylation (Extended Data Fig. 1e). We found that global CA methylation increases within the first 5 days of naive culture conditions, but after day 13 in primed reprogramming (Fig. 1c). Notably, we observed that CH methylation only accumulates upon changing cells to naive or primed culture conditions, concomitant with increased DNMT3B expression (Fig. 1c and Extended Data Fig. 1e,f).
Inspection of CG DNA methylation changes at regulatory elements revealed stepwise changes during primed reprogramming, but only one major change during naive reprogramming between days 7 and 13 (Fig. 1d). Fuzzy clustering identified five distinct classes of dynamic methylation at regulatory elements (Fig. 1e and Supplementary Table 2), with methylation changes generally occurring after, and being inversely correlated with, the expression change of linked genes (Fig. 1e and Extended Data Fig. 1g,h). This suggests that methylation changes at regulatory elements do not drive expression change during reprogramming but maintain repression, similar to reprogramming in mouse cells23.
We then identified the transcription factor motifs associated with methylation changes at regulatory elements (Fig. 1f). Elements with increasing methylation during reprogramming (clusters 1–3) were enriched for the AP-1, JUN and FOS motifs, as was the transient cluster (cluster 5), which was also enriched for OCT4–SOX2 motifs (Fig. 1f). This is consistent with human and mouse studies suggesting that transcription factors at somatic enhancers are sequestered to transiently active elements bound by OKSM, which recruits transcription factors away from the loci maintaining somatic cell identity22,23. Demethylated regulatory elements featured OCT4–SOX2 motifs, and were associated with pluripotency genes, where expression increased after day 3 (cluster 4; Fig. 1e,f). Inspection of methylation changes driven by OKSM in fibroblast medium (up to day 7) revealed that 1,030 enhancers but only 39 promoters feature CG methylation loss of more than 20%, with these enhancers being enriched for AP-1 and pluripotency transcription factor motifs (Extended Data Fig. 1i). These time-course methylome profiles reveal that the first wave of epigenetic remodelling at regulatory elements is driven by OKSM, followed by distinct methylation states coincident with transitioning to primed and naive culture conditions.
Emergence of aberrant DNA methylation
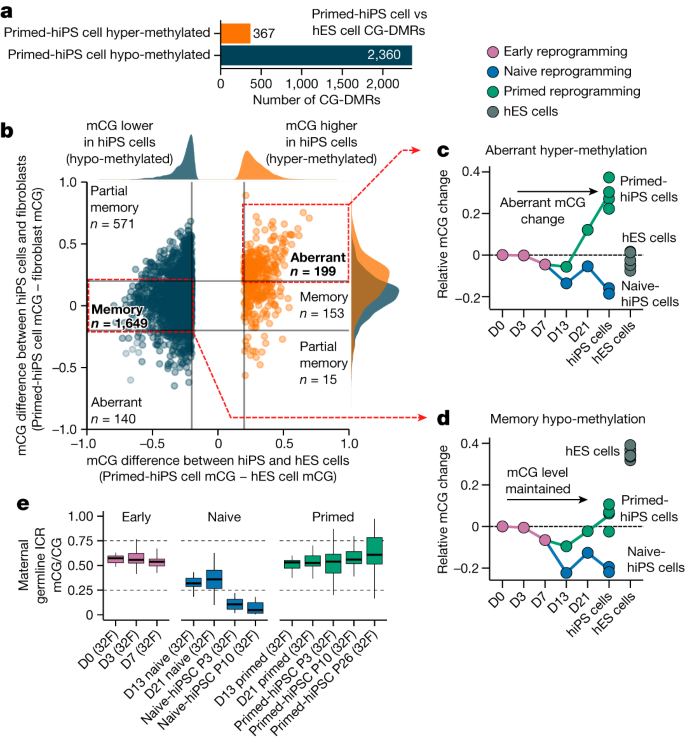
Several reports indicate that hiPS cells feature differentially methylated regions (DMRs) compared with hES cells that can be categorized as either somatic cell epigenetic memory or acquired aberrant methylation states that are unique to hiPS cells, which are not present in the cell of origin or hES cells1,2,3,4,5,7,13,24. Despite reports of DNA methylation differences between hiPS cells and hES cells, their temporal dynamics during reprogramming are not well characterized. We thus first identified CG-DMRs between multiple primed-hiPS cell and hES cell lines (Extended Data Fig. 1j). We identified 2,727 CG-DMRs (methylated CG (mCG)/CG difference >0.2; P ≤ 0.05), with 86.5% showing lower CG methylation levels in hiPS cells (Fig. 2a, Extended Data Fig. 1k and Supplementary Table 3). CG-DMRs could be classified as acquiring aberrant DNA methylation or retaining somatic cell epigenetic memory by comparing the DNA methylation levels between primed-hiPS cells and the fibroblasts that they originated from (Fig. 2b). This revealed that in primed-hiPS cells, 60.4% of the CG-DMRs were hypo-methylated relative to hES cells and showed less than 20% difference in methylation levels relative to fibroblasts, indicating somatic cell epigenetic memory, and an additional 24.2% of the CG-DMRs that were hypo-methylated relative to hES cells harboured higher methylation in primed-hiPS cells relative to fibroblasts, indicating partial epigenetic memory (Fig. 2b). Conversely, a majority of hyper-methylated CG-DMRs (54.2%) exhibited aberrant DNA methylation acquired during reprogramming, with methylation levels more than 20% higher than both fibroblasts and hES cells (Fig. 2b). Time-course analysis revealed that aberrant methylation begins to emerge between days 13 and 21 of primed reprogramming and continues to increase between day 21 and passages 3–10 (Fig. 2c). With memory CG-DMRs, minor transient demethylation (mCG/CG < 0.1) occurred in primed reprogramming (Fig. 2d), concordant with global CG methylation change (Fig. 1b). However, transitioning cells to naive medium triggered substantial demethylation in memory CG-DMRs by day 13 (Fig. 2d,e and Extended Data Fig. 1l,m). For hyper-methylated memory CG-DMRs, we observed demethylation to levels similar to those in hES cells by day 13 (Extended Data Fig. 1l). Overall, we found that aberrant CG methylation does not begin to accumulate upon OKSM induction during early reprogramming, and begins to emerge only after day 13 of primed reprogramming (Fig. 2c). Of note, aberrant CG hyper-methylation loci in primed-hiPS cells were not aberrant in naive reprogramming (Fig. 2c), indicating that aberrant hyper-methylation is a feature of primed and not naive reprogramming.
a, Number of CG-DMRs detected in primed-hiPS versus hES cells. Hypo-methylated CG-DMRs are those that are less methylated in primed-hiPS cells than in hES cells, and hyper-methylated CG-DMRs are those that are more methylated in primed-hiPS cells than in hES cells. b, Relative CG DNA methylation difference at CG-DMRs in primed-hiPS cells versus hES cells (x axis) and fibroblasts (y axis). Each point on the graph represents an individual CG-DMR; blue points represent hypo-methylated DMRs and orange points represent hyper-methylated DMRs. The plot is divided into segments using a cut-off of 0.2 difference in mCG/CG between cell types for classification purposes. Kernel density estimate plots (top and right of the main graph) show the distribution of CG-DMR methylation differences for hypo- and hyper-methylated DMRs. c,d, Time-course of mean CG methylation change across aberrant hyper-methylated CG-DMRs (c) and hypo-methylated memory CG-DMRs (d) relative to the progenitor fibroblast state (day 0). Each point represents mean CG DNA methylation change compared to day 0 for individual samples. The hiPS cell time point includes all passages. e, Methylation at maternal germline ICRs throughout naive and primed reprogramming. In box plots, the horizontal line is the median, the box represents the interquartile range (IQR) and whiskers show either 1.5 × IQR or the data range. n = 1 independent experiment per box plot. ICRs are defined in ref. 21.
We next investigated DNA methylation at imprint control regions (ICRs), which are known to be abnormal in hiPS cells25, with reports indicating that naive culture conditions triggers irreversible methylation loss at ICRs16,20,21. Analysis of CG methylation at known ICRs21,26 revealed that imprints begin losing CG methylation between days 7 and 13, with the full loss of allele-specific methylation not occurring until after day 21 of naive reprogramming (Fig. 2e and Extended Data Fig. 1n). This indicates that demethylation at imprinted loci becomes more extensive the longer cells are cultured in naive conditions, and suggests that imprints may be maintained at day 13 of naive reprogramming.
TNT reprogramming resets the epigenome
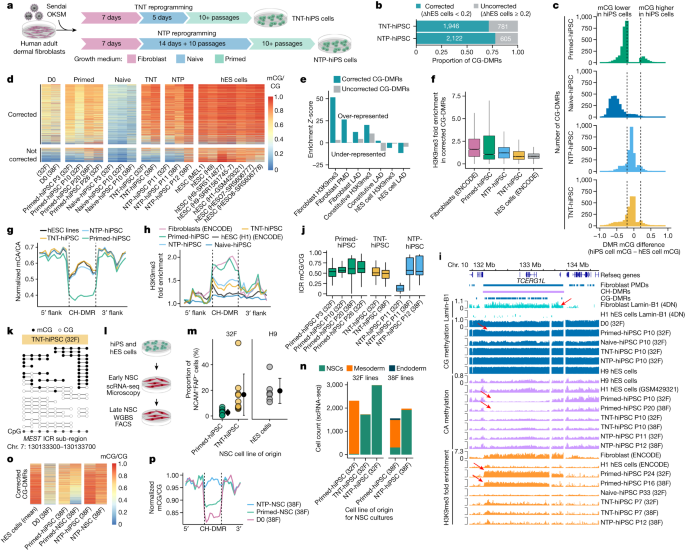
During early development, the pre-implantation embryo undergoes an epigenetic reset involving a wave of global demethylation, during which genomic imprints are protected from demethylation27. By combining our new understanding of epigenomic reconfiguration during reprogramming, we hypothesized that we could avoid somatic cell epigenetic memory and aberrant DNA methylation by reprogramming through a transient naive-like state, similar to the demethylation observed during embryonic development. Thus, we devised two experimental systems. In the first system, we reprogrammed fibroblasts with a transient naive culture treatment for 5 days after the initial 7 days of culturing in fibroblast medium, followed by culturing in primed medium for the remainder of the reprogramming (Fig. 3a), to give rise to transient-naive-treatment hiPS cells (TNT-hiPS cells). In the second system, we first established naive-hiPS cell colonies by extended naive culturing and then transitioned the cells to a primed pluripotent state to give rise to naive-to-primed hiPS cells (NTP-hiPS cells) (Fig. 3a).
a, New reprogramming strategies for TNT-hiPS and NTP-hiPS cells. b, The proportion of CG-DMRs for primed-hiPS cells and hES cells corrected by TNT and NTP reprogramming to a difference of less than 0.2 mCG/CG. c, Differences in DNA methylation between hiPS and hES cells at CG-DMRs. Dashed lines indicate the threshold of 0.2 difference in CG-DMR methylation level. d, Methylation levels in corrected (top) and uncorrected (bottom) CG-DMRs. e, Enrichment permutation testing of corrected and uncorrected CG-DMRs in repressive chromatin. f, H3K9me3 enrichment in corrected CG-DMRs. Primed-hiPS: n = 2; TNT-hiPS: n = 2; NTP-hPSC, n = 3 independent experiments. In box plots, the horizontal line is the median, the box represents the interquartile range (IQR) and whiskers show either 1.5 × IQR or the data range. g, Aggregate profile of CA methylation in hypo-methylated CH-DMRs. Lines represent flank-normalized means. h, H3K9me3 enrichment in hypo-methylated CH-DMRs. Lines represent flank-normalized means. i, Genome track of a CH-DMR intersecting a PMD, fibroblast LAD and a CG-DMR cluster. Arrows indicate partial CG methylation, CA methylation depletion and H3K9me3 enrichment in a fibroblast LAD, as indicated. j, CG methylation in ICRs. The horizontal line is the median, the box represents the IQR and whiskers show either 1.5 × IQR or the data range. n = 1 independent experiment per box plot. k, WGBS reads at the MEST ICR. l, Schematic of NSC differentiation and profiling. m, The proportion of NCAM+FAP− cells during differentiation into NSCs. Primed-hiPS: n = 9; TNT-hiPS: n = 9; H9-hES: n = 6 independent differentiation experiments. Data are mean ± s.d. n, Proportions of different cell types detected in early NSC cultures by single-cell RNA-seq (scRNA-seq). o, Methylation levels in CG-DMRs corrected by NTP reprogramming (as in Fig. 3d) in hiPS cells and derived NSC cultures. p, CG methylation (flank-normalized mCG/CG) in hypo-methylated CH-DMRs in NSCs and progenitor fibroblasts.
We first confirmed that TNT-hiPS cells and NTP-hiPS cells were morphologically and molecularly similar to hES cells (Extended Data Fig. 2a). Testing for genetic aberrations in the hiPS cell lines revealed that two NTP-hiPS cell lines had megabase-scale deletions, and one primed-hiPS cell line had a deletion of about 600 kb, whereas we detected no aberrations in the TNT-hiPS cell lines (Extended Data Fig. 2b). When assessing CG-DMRs detected between primed-hiPS cell and hES cell lines, we observed that a majority of CG-DMRs show epigenetic correction to a state that is highly similar to hES cells for both TNT-hiPS (71.3%) and NTP-hiPS (77.8%) cells (Fig. 3b–d and Extended Data Fig. 2c–f). CG-DMR correction was highly concordant between the TNT and NTP systems (Extended Data Fig. 2c–f). Re-analysis of WGBS data from hiPS cells corrected by SCNT reprogramming13 revealed that TNT-hiPS and NTP-hiPS cells have more CG-DMRs corrected compared to the 59.9% that are corrected in SCNT reprogramming (Extended Data Fig. 2g–i), indicating that TNT reprogramming is more effective at epigenetic correction.
We performed permutation testing to identify the genomic features that show a statistical over- or under-representation of CG-DMRs, revealing that corrected CG-DMRs are highly enriched in regions featuring the repressive histone modification H3K9me3 in fibroblast cells (z-score = 38.9; FDR < 0.01) but depleted in regions of hES cell-specific H3K9me3 (z-score = −4.5; FDR < 0.01; Fig. 3e and Extended Data Fig. 3a). Consistently, corrected CG-DMRs were over-represented in partially methylated domains (PMDs) in fibroblasts (z-score = 25.8; FDR < 0.01; Fig. 3e) and lamina associated domains (LADs)(z-score = 10.6; FDR < 0.01), which are known to co-occur with H3K9me3 in large domains of heterochromatin that are gene-poor, repressive and relate to higher order genome architecture28. We further analysed the relationship between CG-DMRs and repressive chromatin domains by performing H3K9me3 chromatin immunoprecipitation–sequencing (ChIP–seq). Regions enriched for H3K9me3 in fibroblasts that intersect with corrected CG-DMRs showed higher H3K9me3 in primed-hiPS cells compared with TNT-hiPS and NTP-hiPS cells, which were both more similar to hES cells (Fig. 3f), suggesting that repressive chromatin domains featuring epigenetic memory are reset by TNT reprogramming. Another epigenome feature that differs between hiPS cells and hES cells is megabase-scale CH-DMRs, which collectively span 122.3 Mb (4.4%) of the WGBS-mappable genome, and co-occur with cell-of-origin H3K9me34,13. When profiling CH-DMRs (defined in refs. 4,13), we found that CG-DMRs were highly enriched within them (Extended Data Fig. 3a). Moreover, 94.1% of CG-DMRs within CH-DMRs were corrected to an hES cell-like state, compared with 69.0% of CG-DMRs that do not overlap CH-DMRs (Extended Data Fig. 3b). TNT-hiPS and NTP-hiPS cells also showed a greater magnitude of CG methylation correction in CG-DMRs that overlap CH-DMRs (Extended Data Fig. 3c). Inspection of CA methylation in hypo-methylated CH-DMRs (n = 28) revealed that TNT-hiPS and NTP-hiPS cells have a CA methylation profile that is highly similar to hES cells, which is distinct from the low CA methylation levels observed in primed-hiPS cells (Fig. 3g and Extended Data Fig. 3d), in contrast to hyper-methylated CH-DMRs (n = 15; Extended Data Fig. 3e). We observed strong H3K9me3 enrichment in hypo-methylated CH-DMRs for primed-hiPS cells, at levels similar to those in fibroblasts, but TNT-hiPS and NTP-hiPS cells were more similar to hES cells, with markedly less H3K9me3 (Fig. 3h).
As existing hiPS cell lines may feature epigenetic anomalies, we tested whether culturing primed-hiPS cells in naive medium could correct aberrant DNA methylation. We generated primed-to-naive hiPS cells (PTN-hiPS cells) by culturing an established primed-hiPS cell line in naive medium for an extended period, and then transitioned these PTN-hiPS cells back into primed medium to produce primed–naive–primed-hiPS cells (PNP-hiPS cells). Attempts at TNT-like culturing of primed-hiPS cells (5 days in naive medium) caused extensive cell death and spontaneous differentiation when transitioning back to primed medium. PNP-hiPS cells exhibit remethylation and correction of a subset of the CG-DMRs detected between primed-hiPS cells and hES cells (Extended Data Fig. 3f), and show correction of many of the CH-DMRs (Extended Data Fig. 3g). Therefore, PNP reprogramming appears to correct aberrant DNA methylation patterns in primed-hiPS cells, although we observed increased variation in CG methylation at ICRs (Extended Data Fig. 3h). We emphasize that extended culturing of cells in some naive conditions may cause an increase in the frequency of genetic abnormalities16,21; therefore, although epigenetic correction is possible with PNP reprogramming, performing TNT reprogramming is optimal for minimizing genetic abnormalities and disruption of imprinting.
We then tested whether the improved qualities of TNT-hiPS cells result from clonal selection by randomly inserting a known DNA sequence into fibroblasts by lentiviral transduction and then reprogramming them by primed and TNT methods. Cas9-mediated enrichment and nanopore sequencing indicated that TNT-hiPS cells do not result from the selection of rare cell subpopulations (Extended Data Fig. 3i and Supplementary Table 4).
Our results indicate that large repressive chromatin domains associated with the nuclear lamina harbour epigenetic memory in primed-hiPS cells. For example, we detected a 1.7-Mb CH-DMR on chromosome 10 that was enriched for lamin-B1 in fibroblasts but not in hES cells, that also spans a cluster of 175 smaller CG-DMRs, intersects a larger fibroblast PMD and shows more than fivefold enrichment of H3K9me3 in fibroblasts and primed-hiPS cells, but not in TNT-hiPS and NTP-hiPS cells (Fig. 3i). Notably, aberrant epigenomic states in this large domain as well as other domains have been previously observed in primed-hiPS cells using a variety of progenitor cells and reprogramming methods4,6,13. The correction of CG and CH methylation and H3K9me3 in TNT-hiPS and NTP-hiPS cells demonstrates that the majority of epigenetic memory in hiPS cells can be corrected, and suggests that TNT reprogramming reorganizes chromatin architecture beyond what is achieved in conventional reprogramming. This reorganization may affect OKSM-mediated epigenome remodelling, as repressive chromatin domains are refractory to OKSM binding29.
We then assessed the reproducibility of DMRs between studies, observing that even when processed with identical methods, the locations and number of CG-DMRs varies between studies (Extended Data Fig. 4a,b). However, the enrichment of CG-DMRs in repressive chromatin and CH-DMRs was similar across studies (Extended Data Fig. 4c,d,f). When assessing CA methylation using an identical set of CH-DMRs, we observe consistent reproducibility (Extended Data Fig. 4e,f). Principal component analysis revealed that principal component 1 (PC1) and PC2 captured study-dependent differences, whereas PC3 separated primed-hiPS cells and hES cells for all studies, and showed that TNT-hiPS cells were more similar to hES cells by this measure (Extended Data Fig. 4g–i).
Previous studies indicate that naive culturing triggers the loss of genomic imprinting, which is not recovered upon re-priming16,20,21. By contrast, we observed that TNT-hiPS cells have CG methylation patterns that are indicative of imprinting (Fig. 3j and Extended Data Fig. 5a). Analysis of WGBS reads—representative of single DNA molecules—showed equivalent proportions of unmethylated and methylated molecules at ICRs for TNT-hiPS cells, similar to fibroblasts (Fig. 3k and Extended Data Fig. 5b). This is in contrast to NTP-hiPS cells, in which we observed increased variance in the methylation levels at imprinted loci (Fig. 3j and Extended Data Fig. 5a). These data demonstrate that epigenetic memory erasure in TNT reprogramming can co-occur with maintenance of genomic imprinting. We then examined X chromosome inactivation in hiPS cell lines. CG methylation clustering of hiPS cell lines on the basis of 5-kb windows and promoters showed that none of the primed-hiPS, NTP-hiPS or TNT-hiPS cell lines clustered by hiPS cell type and were distributed among the hES cell lines (Extended Data Fig. 5c,d), indicating that TNT-hiPS and NTP-hiPS cells feature appropriate X chromosome inactivation.
Correction persists through differentiation
Previous studies indicate that epigenetic memory and aberrations in primed-hiPS cells can persist through differentiation1,2,3,4, which could functionally affect the resulting cells. We tested whether CG-DMR correction was maintained by differentiating primed-hiPS, TNT-hiPS and NTP-hiPS cells into neural stem cells (NSC) (Fig. 3l). We observed that NSC cultures derived from primed-hiPS cells produce many fibroblast-like cells in the early NSC cultures, similar to endoderm differentiation30. Notably, these fibroblast-like cells did not emerge when differentiating TNT-hiPS and NTP-hiPS cells (Extended Data Fig. 5e). FACS quantification of NCAM+FAP− cells in the differentiating culture revealed that TNT-hiPS cells differentiate more efficiently into NSCs, at a rate similar to hES cells (Fig. 3m). We characterized these cultures by scRNA-seq, revealing that early NSC cultures from fibroblast-derived primed-hiPS cells (which are of mesoderm origin) consist of 75.9–98.7% mesoderm-like cells (defined by the markers BMP4, HAND1 and TGFB1), which were absent from NSC cultures generated from fibroblast-derived TNT-hiPS cells (0.35%) and NTP-hiPS cells (0.06–0.27%) (Fig. 3n and Extended Data Fig. 5f). After clearing the NSC cultures of fibroblast-like cells (by passaging at least 6 times), we performed WGBS profiling of the remaining NSCs to assess maintenance of corrected epigenetic states through differentiation. Whereas the hypo-methylation persisted at CG-DMRs in primed-hiPS cell derived NSCs, epigenetic correction was maintained for NSCs derived from NTP-hiPS cells (Fig. 3o). We then assessed CH-DMRs to inspect partial CG methylation, reflective of a PMD state, as this would suggest transmission of repressive chromatin of fibroblast origin. NSCs derived from primed-hiPS cells indeed maintained partial CG methylation, in contrast to NTP-hiPS cells, which showed high CG methylation levels suggestive of remodelling of repressive chromatin (Fig. 3p). These results indicate that epigenetic memory in primed-hiPS cells impairs differentiation efficiency and persists through differentiation.
Isogenic evaluation of hiPS and hES cells
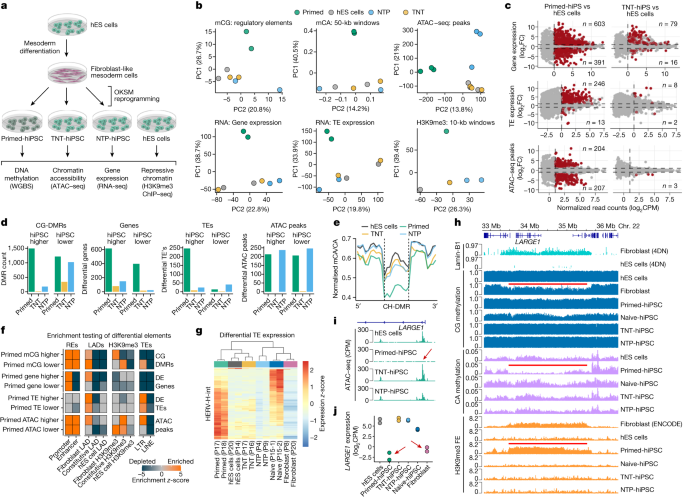
Up to this point, we have shown that TNT reprogramming epigenetically resets hiPS cells to a molecular state that is more similar to hES cells. However, previous reports suggest that genetic background variation may confound comparisons of pluripotent cell lines31,32, including comparisons of hiPS cells and hES cells11. Therefore, we designed a series of isogenic reprogramming experiments to unambiguously compare hiPS cells and hES cells. We first differentiated hES cells into secondary fibroblast-like cells11 and confirmed that they were CD90+TRA160− and clustered with primary fibroblast lines based on CG methylation (Extended Data Fig. 6a–c). We then reprogrammed these secondary fibroblasts using the primed-hiPS, TNT-hiPS and NTP-hiPS cell protocols and performed WGBS, RNA-seq, assay for transposase-accessible chromatin with sequencing (ATAC–seq) and H3K9me3 ChIP–seq (Fig. 4a).
a, Experimental design for differentiating hES cells to fibroblast-like cells and then reprogramming them to hiPS cells using the primed, TNT and NTP methods. b, Principal component analysis of CG methylation at GeneHancer elements, mCA/CA of 50-kb genome windows, normalized ATAC–seq read counts in peaks, normalized global gene expression, normalized global transposable element (TE) expression and normalized H3K9me3 ChIP–seq read counts. Data were quantile-normalized counts per million (CPM). c, Differential-testing MA plots for gene expression (determined by RNA-seq), TE expression (RNA-seq), and chromatin accessibility (ATAC–seq) for hiPS cells versus hES cells. Red points indicate FDR <0.05. Numbers on plots enumerate the ‘up’ or ‘down’ significant-features counts for each comparison. d, Differential testing of hES cells versus hiPS cell types for CG-DMRs, gene expression, TE expression and ATAC–seq peaks. ‘hiPS cell higher’ indicates that the value is higher in hiPS cells than in hES cells, and ‘hiPS cell lower’ indicates that the value is lower in hiPS cells than in hES cells. e, Aggregate profile plot of CA methylation levels in hypo-methylated CH-DMRs. f, Permutation testing enrichment (z-scores) of differential elements. z-scores larger than 5 were reduced to 5 for visualization. REs, regulatory elements. g, Relative expression heatmap of HERV-H-int elements that are differentially expressed between hES cells and primed-hiPS cells (n = 167). h, Genome track of a CH-DMR region detected in hES cells versus primed-hiPS cells and associated epigenomic features. Red lines show fibroblast LAD, fibroblast PMD in the primed-hiPS cells and fold enrichment (FE) of H3K9me3 in primary fibroblasts, as indicated. i, Normalized ATAC–seq signal at the LARGE1 promoter. The red arrow highlights the absence of an ATAC–seq peak in primed-hiPS cells. j, Gene expression of LARGE1 in isogenic hES cells, hiPS cells and progenitor fibroblasts. Red arrows indicate repression in primed-hiPS cells and fibroblasts.
To visualize the differences between the isogenic hiPS cells and hES cells, we calculated principal components for global measures of CG and CA methylation, chromatin accessibility, gene and transposable element expression and H3K9me3 enrichment (Fig. 4b). This confirmed that even when controlling for genetic differences, TNT-hiPS cells are consistently highly similar to hES cells, whereas primed-hiPS cells are molecularly distinct. Next, we performed differential testing for CG-DMRs, gene and transposable element expression and ATAC–seq peaks for hES cells versus primed-hiPS, TNT-hiPS and NTP-hiPS cells (Fig. 4c,d). We detected 2,709 CG-DMRs for primed-hiPS cells (mCG difference >0.2; FDR <0.05), and only 358 for TNT-hiPS and 1,200 for NTP-hiPS cells (Fig. 4d, Extended Data Fig. 6d–h and Supplementary Table 5). Moreover, TNT-hiPS and NTP-hiPS cells also showed CA methylation levels in CH-DMRs similar to their origin hES cells, contrary to primed-hiPS cells (Fig. 4e and Extended Data Fig. 6i).
We identified 994 genes that were differentially expressed between isogenic primed-hiPS cells and hES cells (log2-transformed fold change (FC) > 1, FDR <0.05), however these differences were largely ameliorated in TNT-hiPS and NTP-hiPS cells, with only 95 and 165 genes being differentially expressed, respectively (Fig. 4c,d, Extended Data Fig. 7a and Supplementary Table 6). When assessing the relationship between differential gene expression and promoter CG-DMRs, we observed that differential methylation is associated with gene-expression change (Extended Data Fig. 7b and Supplementary Table 7). For primed-hiPS cells, 172 out of 547 (31.4%) of promoter CG-DMRs showed associated differential expression, whereas only 49 out of 215 (22.7%) of promoter CG-DMRs in TNT-hiPS cells had linked gene-expression differences. Gene ontology analyses revealed that genes that were differentially expressed in primed-hiPS cells are enriched for mesoderm development, among other terms (Supplementary Table 6). We then profiled the expression of genes with mesoderm-related ontologies, revealing that TNT-hiPS cells cluster more closely with hES cells than primed-hiPS cells (Extended Data Fig. 7c). Early mesoderm differentiation markers for WNT signalling (WNT5A, WNT3 and WNT11) and mesoderm progenitor markers (BMP4, MESP1 and FOXC1) showed increased expression in primed-hiPS cells compared with hES cells, which is largely corrected in TNT-hiPS cells (Extended Data Fig. 7d). Inspection of fibroblast-specific genes that retain their expression in primed-hiPS cells showed that primed-hiPS cells feature a gene-expression signature with elements of the fibroblast state that are not observed in TNT-hiPS or NTP-hiPS cells (Extended Data Fig. 7e), further demonstrating that the molecular memory of the cell of origin in primed-hiPS cells is corrected by TNT reprogramming.
When testing for differences in chromatin accessibility, we observed 411 differential ATAC–seq peaks between hES cells and primed-hiPS cells, whereas only 3 peaks were different between hES cells and TNT-hiPS cells, making them practically indistinguishable (log2FC > 2, FDR <0.05; Fig. 4c,d and Extended Data Fig. 8a,b). NTP-hiPS cells exhibited 483 differential peaks, but not the same direction as primed-hiPS cells (Fig. 4d and Extended Data Fig. 8a). Motif analysis showed that primed-hiPS cells lack accessibility at loci enriched for OKSM binding motifs, and regions with uniquely accessible chromatin in primed-hiPS cells are enriched for transcription factors associated with differentiation (Extended Data Fig. 8c).
For genomic imprinting, TNT-hiPS cells did not show extensive demethylation at ICRs, in contrast to NTP-hiPS cells, which more closely resembled naive-hiPS cells (Extended Data Fig. 8d), consistent with previous reports of naive cultured hES cells showing imprinting loss when re-primed20. Clustering analysis based on imprinted gene expression also showed that TNT-hiPS cells were more similar to hES cells than NTP-hiPS cells (Extended Data Fig. 8e), and differential expression testing indicated imprinting loss in NTP-hiPS cells, but not in TNT-hiPS cells, for genes including PEG3, MEG3 and KCNQ1 (Supplementary Table 6). Moreover, when examining the relationship between CG methylation at ICRs with the change in expression of the linked imprinted gene, NTP-hiPS cells showed the greatest loss of imprinting at the expression level, with TNT-hiPS cells being the most similar to hES cells (Extended Data Fig. 8e,f). This further demonstrates that loss of imprinting is caused by extended naive culturing and can be avoided with TNT reprogramming.
As transposable element expression signatures are characteristic of different pluripotent cell states20,33,34,35, we next tested for differential abundance of transposable elements between hES cells and hiPS cells. We identified 246 up-regulated and 13 down-regulated transposable elements in primed-hiPS cells (log2FC >1, FDR <0.05; Fig. 4c,d). Notably, these differences were almost completely abolished by TNT reprogramming, with only 8 up- and 2 down-regulated transposable elements, whereas NTP-hiPS cells still showed 65 differentially expressed transposable elements (Fig. 4c,d, Extended Data Fig. 8g and Supplementary Table 8). We further found that genes within 50 kb of up-regulated transposable elements frequently showed upregulation in primed-hiPS cells, but not in TNT-hiPS or NTP-hiPS cells (Extended Data Fig. 8h). We also observed enrichment of primed-hiPS cell ATAC–seq peaks at long terminal repeat (LTR) transposable elements, co-occurring with reduced CG methylation (Fig. 4f). Closer inspection revealed that the up-regulated transposable elements in primed-hiPS cells are predominantly human endogenous retrovirus subfamily H (HERV-H) elements (80%, 197 out of 246) and their flanking LTR7 sequences, and that primed-hiPS cells express distinct copies of these elements compared with those expressed in naive-hiPS cells (Fig. 4g, Extended Data Fig. 8i and Supplementary Table 8). This is exemplified by the up-regulated HERV-H-int_dup2429 copy in primed-hiPS cells, featuring reduced DNA methylation and a 5′ ATAC–seq peak, neither of which are present in the hES or TNT-hiPS cells (Extended Data Fig. 8j). We further validated our observations that transposable element expression is also different between hiPS cells and hES cells by performing the same transposable element differential expression analyses on two published RNA-seq datasets11,13 (Extended Data Fig. 8k,l). We observed that transposable element expression in primed-hiPS cells can be partially corrected by SCNT reprogramming (Extended Data Fig. 8l), further demonstrating that dysregulation of transposable elements can be avoided by enhanced epigenome-resetting approaches13. The correction of abnormal transposable element expression is important, as it may contribute to the phenotypic heterogeneity of hiPS cells and could lead to mutagenesis36, and increased HERV-H expression can inhibit hiPS cell differentiation efficiency37.
When analysing the relationship between differential DNA methylation, gene expression and chromatin states, we observed that fibroblast-associated repressive chromatin domains were highly enriched for the elements that we identify as significantly different in primed-hiPS cells (Fig. 4f). When inspecting an approximately 2-Mb fibroblast LAD on chromosome 22, we observed that primed-hiPS cells had a PMD with concomitant H3K9me3 enrichment similar to the fibroblast cells, but distinct from isogenic TNT-hiPS cells, NTP-hiPS cells and hES cells (Fig. 4h). Moreover, within this fibroblast LAD, the LARGE1 promoter showed no chromatin accessibility in primed-hiPS cells, coupled with strong transcriptional repression (Fig. 4i,j), also exemplified by the MYH14–KCNC3 locus (Extended Data Fig. 9a). These examples highlight that lamina-associated megabase-scale regions of repressive chromatin that are present in differentiated cells are retained in primed-hiPS cells, but can be reset by reprogramming through the naive state. To further validate the ability of TNT reprogramming to produce hiPS cells that more closely resemble hES cells than those produced by conventional reprogramming, we evaluated published criteria6,38,39 for using DNA methylation and gene-expression signatures for selecting good hiPS cell clones, which indicated that TNT-hiPS cells would produce better hiPS cells for differentiation (Extended Data Fig. 9b–e).
Improved differentiation of TNT-hiPS cells
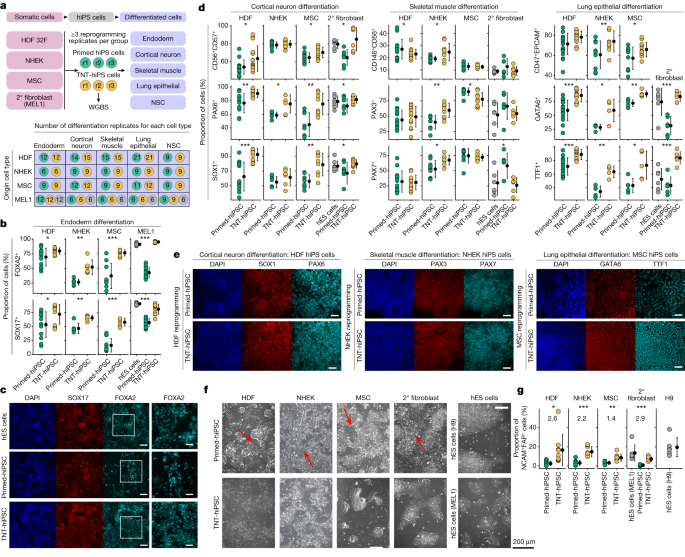
Substantial evidence indicates that epigenetic memory in iPS cells affects differentiation; however, the functional differences between iPS cells and ES cells remain topics of debate1,2,3,11. Therefore, we generated additional independent hiPS cell lines that were reprogrammed from primary human dermal fibroblasts (HDFs), keratinocytes (NHEK cells), mesenchymal stem cells (MSCs) and our hES cell-derived isogenic secondary fibroblasts to comprehensively test for differences in primed and TNT-hiPS cell differentiation capacity (Fig. 5a). We reprogrammed each origin somatic cell type in triplicate to produce both TNT-hiPS and primed-hiPS cells and then differentiated each hiPS cell line into definitive endoderm, cortical neurons, skeletal muscle cells, lung epithelial cells and neural stem cells.
a, Experimental design for multi-lineage primed and TNT reprogramming and differentiation into five cell types. Top, the four somatic cell lines reprogrammed into primed-hiPS cells and TNT-hiPS cells with three independent reprogrammings (r1–r3) performed per group, and with each subsequently differentiated into five different cell types, with independent replication. Bottom, the number of independent differentiation replicates performed for origin cell types (rows) and differentiated cell types (columns). Coloured circles represent primed-hiPS cell (green), TNT-hiPS cell (yellow) and hES cell (grey). 2° fibroblasts, secondary fibroblasts. b, Endoderm differentiation quantification for hiPS cells derived from secondary fibroblasts, showing the proportion of cells positive for FOXA2 and SOX17 by immunofluorescence analysis. c, Representative images from immunofluorescence analysis of FOXA2 and SOX17 in endoderm differentiation of hiPS cells derived from secondary fibroblasts. The outlined region is enlarged on the right. Scale bars, 100 μm (main image), 50 μm (enlarged region). d, Quantification of multi-lineage cell differentiation in hiPS cell lines by FACS and immunofluorescence analyses using CD56, CD57 (FACS), PAX6 and SOX1 (immunofluorescence) for cortical neuron differentiation, CD146, CD56 (FACS), PAX3 and PAX7 (immunofluorescence) for skeletal muscle differentiation, and CD47, EPCAM (FACS), GATA6 and TTF1 (immunofluorescence) for lung epithelial differentiation. e, Representative images from immunofluorescence analysis of cell differentiation using SOX1 and PAX6 for cortical neurons, PAX3 and PAX7 for skeletal muscle, and GATA6 and TTF1 for lung epithelial cells. Scale bars, 50 μm. f, Phase-contrast images taken four days after passaging plated embryoid bodies during differentiation into NSCs. Large stretched-out fibroblast-like cells are evident during differentiation from primed-hiPS cells (red arrows). g, The percentage of NCAM+FAP− cells (from FACS analysis) after plating of embryoid bodies during NSC differentiation. log2FC values are shown on the graph. d,g, Data are mean ± s.d; two-sided t-test for primed versus TNT; ***P < 0.0001, **P < 0.001, *P < 0.05. Details of replication are presented in Methods, ‘Statistics and reproducibility’.
We first performed WGBS and tested for CG-DMRs between primed and TNT-hiPS cells for each origin cell type to identify epigenetic differences that are not confounded by genetic differences. Clustering of samples on the basis of CG methylation in DMRs revealed that, irrespective of origin cell type, TNT-hiPS cells consistently cluster with hES cells, whereas primed-hiPS cells cluster more closely with their origin cells (Extended Data Fig. 9f). We again observed that CA methylation in TNT-hiPS cells was more similar to hES cells at CH-DMRs that are hypo-methylated in primed-hiPS cells, but note that the magnitude of difference for CA methylation between primed and TNT-hiPS cells from NHEK cells and MSCs was less than that observed for those from HDFs (Extended Data Fig. 9g). Testing for differences in CG methylation at ICRs revealed no differences between primed-hiPS and TNT-hiPS cells for reprogrammed HDFs, whereas TNT-hiPS cells from MSCs showed increased CG methylation at two ICRs, and at 15 out of 67 for hiPS cells reprogrammed from keratinocytes, although 8 of these were in a single cluster of secondary ICRs (Extended Data Fig. 9h). Despite the cell-of-origin-dependent differences, which may be due to different initial epigenomes and reprogramming kinetics, the DNA methylation differences between these additional primed-hiPS and TNT-hiPS cells were broadly consistent with the previously analysed lines (Figs. 3 and 4).
We then extensively tested the differentiation capacity of all these hiPS cell lines by FACS and immunofluorescence quantification (Fig. 5a, Extended Data Fig. 10, Supplementary Tables 9 and 10 and Supplementary Data 1). When assessing definitive endoderm differentiation, we observed that TNT-hiPS cells were consistently more efficient in differentiating into definitive endoderm compared with primed-hiPS cells, irrespective of the origin cell type (Fig. 5b,c and Extended Data Fig. 10b–d). Moreover, TNT-hiPS cells generated from secondary fibroblasts derived from hES cells, primary HDFs and MSCs differentiated more efficiently than primed-hiPS cells into both cortical neurons and lung epithelial cells, which both showed a greater proportion of cells expressing key markers of these cell types (Fig. 5d,e and Extended Data Fig. 10a–d; Methods). For skeletal muscle cell differentiation, both TNT-hiPS and primed-hiPS cells generated from MSCs, HDFs and secondary fibroblasts differentiated at similar efficiencies (Fig. 5d,e and Extended Data Fig. 10a–d; Methods). In the case of NHEK-derived hiPS cells, both primed-hiPS and TNT-hiPS cells differentiated at a similar efficiency into cortical neurons, but TNT-hiPS cells were more efficient at differentiating into lung epithelial cells and skeletal muscle cells than primed-hiPS cells (Fig. 5d,e and Extended Data Fig. 10c,d). Finally, during early differentiation into NSCs, when NSC colonies were forming, we again observed the spontaneous appearance of elongated fibroblast-like cells when the cells were derived from primed-hiPS cells, but not when they were derived from TNT-hiPS cells (Fig. 5f). Quantification of NSC differentiation efficiency showed that the proportion of NSCs (NCAM+FAP−) was consistently higher in cultures derived from TNT-hiPS cells than those derived from primed-hiPS cells and closer to the differentiation efficiency observed for hES cell lines (Fig. 5g). These reprogramming and differentiation experiments provide strong evidence that the epigenetic differences in primed-hiPS cells are associated with reduced differentiation capacity that can be attenuated by TNT reprogramming.
Discussion
Our characterization of naive and primed reprogramming dynamics enabled new insights into the nature of epigenetic remodelling in iPS cells, guiding the development of the TNT reprogramming strategy. Our study extends previous work1,2,3,4,13 by showing that epigenetic memory is concentrated in repressive chromatin domains from the cell of origin marked by H3K9me3, that are associated with the nuclear lamina in the origin cell type. We found that TNT reprogramming effectively erases epigenetic memory, particularly in regions of chromatin–lamina interactions, and improves differentiation. If a cell’s response to differentiation cues depends on how chromatin is spatially organized to make loci available for transcription factor binding40, the differentiation bias in primed-hiPS cells may be due to heterochromatic memory influencing transcription factor binding dynamics.
The more complete epigenome reset achieved through TNT reprogramming suggests that this strategy may mimic aspects of the epigenetic reset that occurs during human pre-implantation development. First, TNT reprogramming remodels H3K9me3 heterochromatin, which also occurs during early embryonic development before lineage-specific H3K9me3 is established post-implantation41. Second, TNT reprogramming facilitates transient genome-wide demethylation, similar to pre-implantation development42. Third, genomic imprints are protected from erasure during pre-implantation epigenome resetting, and our data indicate that the transient nature of TNT reprogramming can minimize loss of imprinting, as imprinting loss appears to be symptomatic of extended culturing in naive medium.
Our observation that HERV-H transposable elements show higher expression in primed-hiPS cells compared with hES cells—but not in TNT-hiPS cells—is particularly important, as aberrant HERV-H transcription has been reported to increase the chance of L1 transposable element mRNA expression initiated from HERV-H promoters, leading to mutagenesis in hiPS cells43. Previous studies suggest that transcriptional and epigenetic signatures present in hiPS cells can be donor-dependent, even in isogenic systems11,44,45. Here we independently verified that isogenic primed-hiPS cells and hES cells exhibit significant differences in gene expression, but further demonstrated that these differences can be abolished through TNT reprogramming. This indicates that the epigenome has an important role in driving the differences between hES cells and hiPS cells. Moreover, our differentiation experiments demonstrate that genetically matched TNT-hiPS cells have an enhanced and more homogeneous differentiation potential than primed-hiPS cells.
By leveraging the TNT reprogramming system, we have revealed the functional benefit of more completely resetting the epigenome. Prior to this work, SCNT reprogramming was the only method shown to improve DNA methylation anomalies13. However, SCNT-reprogrammed cells can still feature persistent cell-of-origin H3K9me3 heterochromatin46, and the technique is difficult and unfeasible to scale. Our work shows that TNT reprogramming is a practical and scalable approach to overcome these intrinsic characteristics of hiPS cells, which is important for the clinical delivery of this technology. As TNT reprogramming enables high-fidelity resetting of the epigenome and transcriptome along with improved differentiation, we view this as a powerful model system for studying epigenetic memory and the mechanisms maintaining cell-of-origin heterochromatin.
Methods
Cell culture
All cell lines used and derived by different approaches in this study are listed in Supplementary Table 1. Detailed information about the experimental design, materials and reagents is presented in the Reporting Summary. Primary human adult dermal fibroblasts (HDFa) from three different female donors were obtained from Gibco (C-013-5C, lot no. 1029000 for 38F and lot no. 1569390 for 32F) and cultured following the manufacturer’s recommendations. In brief, cells were thawed and plated into flasks in Medium 106 (Gibco) supplemented with low serum growth supplement (LSGS) (Gibco) for expansion. Cells were cultured in a 37 °C, 5% O2 and 5% CO2 incubator, and the medium was changed every other day. The use of human embryonic stem cells (H9 and MEL1) was carried out in accordance with approvals from Monash University and the Commonwealth Scientific and Industrial Research Organisation (CSIRO) Human Research Ethics Offices. Conventional primed-hiPS cells and H9 hES cells (WiCell Research Institute; http://www.wicell.org) were maintained as described in the below section. The cell lines used in this study were regularly tested and were mycoplasma negative. Human dermal fibroblasts and NHEKs were authenticated by ThermoFisher and Lonza, respectively, as per description in the CoA. hES cells were authenticated in the Laslett lab. MSCs were authenticated in the Heng lab. These cell lines were also routinely authenticated in-house via morphological assessment, immunofluorescence for identity markers, or RNA-seq.
Cell culture media
Fibroblast medium: DMEM (ThermoFisher), 10% fetal bovine serum (FBS, Hyclone), 1% non-essential amino acids (ThermoFisher), 1 mM GlutaMAX (ThermoFisher), 1% penicillin-streptomycin (ThermoFisher), 55 μM β-mercaptoethanol (ThermoFisher) and 1 mM sodium pyruvate (ThermoFisher). Naive medium (t2iLGoY)19: 50:50 mixture of DMEM/F12 (ThermoFisher) and neurobasal medium (ThermoFisher), supplemented with 2 mM L-glutamine (ThermoFisher), 0.1 mM β-mercaptoethanol (ThermoFisher), 0.5% N2 supplement (ThermoFisher), 1% B27 supplement (ThermoFisher), 1% penicillin-streptomycin (ThermoFisher), 10 ng ml−1 human leukaemia inhibitory factor (made in-house), 250 μM L-ascorbic acid (Sigma), 10 μg ml−1 recombinant human insulin (Sigma), 1 μM PD0325901 (Miltenyi Biotec), 1 μM CHIR99021 (Miltenyi Biotec), 2.5 μM Gö6983 (Tocris), 10 μM Y-27632 (Abcam). Primed hiPS cell medium (KSR/FGF2): DMEM/F12 (ThermoFisher), 20% knockout serum replacement (KSR) (ThermoFisher), 1 mM GlutaMAX (ThermoFisher), 0.1 mM β-mercaptoethanol (ThermoFisher), 1% non-essential amino acids (ThermoFisher), 50 ng ml−1 recombinant human FGF2 (Miltenyi Biotec), 1% penicillin-streptomycin (ThermoFisher). Primed hiPS cell medium (Essential 8 (E8)): 10 ml of E8 supplement (Gibco) to 500 ml medium basal (Gibco), supplemented with 1% penicillin-streptomycin (Gibco).
Derivation of TNT-hiPS cells and NTP-hiPS cells
Human somatic cell reprogramming was performed as previously described16,22,48. In brief, early passages (<P6) fibroblast cells were seeded into 6-well plates at 50,000–70,000 cells per well before transduction in fibroblast medium. Cells in one well were trypsinized for counting to determine the volume of virus required for transduction (multiplicity of infection), and transduction was performed using the CytoTune 2.0 iPSC Sendai Reprogramming Kit (Invitrogen) consisting of four transcription factors (OCT4, SOX2, MYC and KLF4). Twenty-four hours later, the medium was removed, with subsequent medium changes performed every other day. For the derivation of primed-hiPS cells, cells were reseeded onto a layer of iMEFs on day 7 of reprogramming and transitioned to primed medium (KSR/FGF2 or E8 on vitronectin; Supplementary Table 1) on the next day. The cells were cultured to confluency (around day 18–21 of reprogramming) and further passaged with Collagenase IV (ThermoFisher) for cell line establishment. For derivation of TNT-hiPS cells, the day 7 reprogramming intermediates were transitioned to naive medium (t2iLGoY) instead. When dome-shaped colonies were evident 5 days later, intermediate cells were collected using Accutase (Stem Cell Technologies) and reseeded onto a layer of iMEFs in naive conditions. The medium was switched to primed medium (KSR/FGF2 or E8; Supplementary Table 1) the following day. When the culture became confluent, cells were collected using collagenase IV and maintained in primed medium (KSR/FGF2 or E8; Supplementary Table 1) on iMEFs. Cells were cultured in a 37 °C, 5% O2 and 5% CO2 incubator with daily medium change. Cells are usually passaged every 4–5 days. For derivation of NTP-hiPS cells: after 16–18 days post-transduction (8–10 days in naive condition), naive-hiPS cells were collected using Accutase (Stem Cell Technologies) and passaged more than 10 times. The established naive-hiPS cells were confirmed by flow cytometry and immunostaining for naive pluripotency-associated markers. Naive-hiPS cells were then collected using Accutase (Stem Cell Technologies) and reseeded in naive condition, the medium was then switched to Primed hiPSC medium (E8) the following day. When the culture became confluent, cells were collected using Collagenase IV (ThermoFisher) and maintained in Primed hiPSC medium (E8). Cells were cultured in 37 °C, 5% O2 and 5% CO2. All cell lines were tested by CGH array and reported normal.
Estimations of cell diversity by Cas9 enrichment for lentivirus insertion mapping
To prepare enriched Oxford Nanopore Technologies (ONT) sequencing libraries, we used PoreChop to design 2 guide RNAs (gRNAs) (5′-AGATCCGTTCACTAATCGAATGG-3′ and 5′-GGAACAGTACGAACGCGCCGAGG-3′) for Cas9-mediated cleavage approximately 1 kb within each end of the integrated lentiviral sequences. These gRNAs were designed to not match elsewhere in the hg38 human reference genome. We confirmed their on-target efficiency by Cas9 (IDT: Alt-R S.p. Cas9 Nuclease V3; catalogue no. 1081058) cleavage of the lentiviral DNA, visualized on gel, in a separate experiment. DNA dephosphorylation (NEB: Quick CIP; M0525S), single guide (IDT: Alt-R CRISPR–Cas9 CRISPR RNA (crRNA) and Alt-R CRISPR–Cas9 trans-activating crRNA (tracrRNA); catalogue no. 1072532) and RNP formation, Cas9 cleavage and subsequent library preparation (ONT: SQK-CS9109) were largely performed according to the ONT Cas9 enrichment guidelines. We increased the starting amount of DNA to 5 µg, and the dephosphorylation and cleavage incubation times to 2 h and 24 h, respectively. For two replicates of each reprogramming method, we then loaded 350 ng of the enriched DNA library onto a MinION R9.4 flow cell, as per the manufacturer’s recommendations, and sequenced for 48 h. Additionally, for the 32F fibroblast sample, 3 µg of unenriched DNA was sequenced on a PromethION R9.4 flow cell (library prep kit SQK-LSK110) by the Kinghorn Centre for Clinical Genomics (KCCG). For data analysis, reads with a Phred score ≥10 were basecalled with Guppy (version 5.0.11). These reads were mapped with minimap2 (version 2.17) to both the human reference genome (hg38), and the sequence of the expected lentiviral insert49. Alignment maps were filtered with samtools (version 1.13) to only keep primary alignments with a length ≥800 bp, and a mapping quality50 of 60. Reads that mapped to both hg38 and the lentivirus sequence were retained and then subjected to another round of filtering. Here, reads were discarded when the base pair interval between the alignments to the lentiviral sequence and hg38 on the read was ≥51 bp. Reads that originated from the unenriched library and comprised a complete (≥4,500 bp) putative lentiviral insert, spanned by a genomic alignment, as identified by TLDR (version 1.2.2) were kept51. Exact insert sites per read were identified based on the coordinates of both alignment maps (hg38 and lentiviral) to the original read. Exact insert sites were clustered together with bedtools (version 2.30.0) cluster within a 50-bp interval52. For each cluster, the coverage was calculated and the smallest start and largest end coordinates were selected as the exact insert site.
The diversity of cell populations was estimated by a Poisson bootstrap53. Here, we model a Poisson distribution of total insertion landscape based on the sequencing coverage of unique lentiviral insert sites. This model infers the amount of non-sequenced insertion sites, which in return is used to adapt the model until convergence, and results in an estimate for the lentiviral insertion diversity.
Secondary fibroblast reprogramming system
hES cells were cultured in fibroblast medium without FGF2 containing DMEM, 10% FBS, 1 mM L-glutamine, 100 µM MEM non-essential amino acids, and 0.1 mM β-mercaptoethanol, for a week. Cells were passaged three times using 0.25% trypsin and then sorted for THY1+TRA160− populations.
Neural stem cell differentiations
hiPS cells were cultivated in E8 medium (Life Technologies) on Cultrex (R&D Systems) coated TC dishes and split 1∶10 every 5 days. Colonies were mechanically disaggregated with 0.5 mM EDTA in PBS (Sigma). After splitting, pieces of colonies were collected by sedimentation and resuspended in E8 medium with 10 μM ROCK inhibitor (Selleckchem) and cultured in petri dishes to form embryoid bodies in suspension. After 24 h, the medium was changed to Knockout DMEM (Life Technologies) with 20% Knockout Serum Replacement (Life Technologies), 1 mM β-mercaptoethanol (Sigma), 1% non-essential amino acids (NEAA, Life Technologies), 1% penicillin/streptomycin (Life Technologies) and 1% Glutamax (Life Technologies) supplemented with 10 µM SB-431542 (Selleckchem), 1 µM dorsomorphin (Selleckchem) for neural induction, as well as 3 µM CHIR99021 (Cayman Chemical) and 0.5 µM PMA (Sigma). Medium was replaced on day 3 by N2B27 medium (50% DMEM-F12 (Life Technologies), 50% Neurobasal (Life Technologies) with 1∶200 N2 supplement (R&D Systems), 1∶100 B27 supplement lacking vitamin A (Miltenyi Biotec) with 1% penicillin-streptomycin (Life Technologies) and 1% Glutamax (Life Technologies)) supplemented with the same small molecule supplements. On day 4, SB-431542 and dorsomorphin were withdrawn and 150 µM ascorbic acid (Sigma) was added to the medium. On day 6, the embryoid bodies were triturated with a 1,000 µl pipette into smaller pieces and plated on Cultrex-coated 12-well plates at a density of about 10–15 per well in NSC expansion medium (N2B27 with CHIR, PMA, and ascorbic acid). After another 5 days, cells were split at a ratio of 1:5 using Trypsin-EDTA (Life Technologies) and Trypsin inhibitor (Sigma) onto a new Cultrex-coated well. After another 5 days, cells were collected by 10 min trypsinization at 37 °C to generate a single-cell suspension for scRNA-seq workflow.
Endoderm progenitor differentiation
The endoderm differentiation was adapted and performed as previously described54,55. In brief, hiPS cells were collected and replated onto plates coated with Matrigel and cultured in primed hiPS cell medium (KSR/FGF2) with medium change for an additional day before differentiation. To differentiate into endodermal progenitor cells, the cells were cultured in chemically defined medium containing 100 ng ml−1 activin A, 20 ng ml−1 FGF2, 10 ng ml−1 bone morphogenetic factor 4 (BMP4), and 10 µM LY294002 for 3–4 days and assessed for differentiation efficiency.
Cortical neuron differentiation
hiPS cells were seeded onto flasks coated with Matrigel at a density of 0.5–1 × 104 cells per cm2 in primed hiPS cell medium (KSR/FGF2). After 48 h, the medium was changed to neural induction medium containing DMEM/F12, B27 without vitamin A supplement (Gibco, ThermoFisher Scientific), N2 supplement (Gibco, ThermoFisher Scientific), 0.1% β-mercaptoethanol (Gibco, ThermoFisher Scientific), 0.66% bovine serum albumin (Sigma-Aldrich), 1% sodium pyruvate (Gibco, ThermoFisher Scientific), 1% non-essential amino acids (Gibco, ThermoFisher Scientific), 1% penicillin and streptomycin, 100 ng ml−1 LDN193189 (Tocris Bioscience, Bio-Techne) for 14 days.
Skeletal muscle cell differentiation
hiPS cells were seeded onto flasks coated with Matrigel at a density of 0.5–1 × 104 cells per cm2 in primed hiPS cell medium (KSR/FGF2). After 24 h, medium was changed to DMEM/F12-based medium supplemented with ITS (insulin + transferrin + selenium; Sigma-Aldrich) with 1% penicillin and streptomycin (Gibco, ThermoFisher Scientific), 3 µM CHIR99021 (Miltenyi Biotec), 0.5 µM LDN193189 (Tocris Bioscience, Bio-Techne) for 3 days. On days 4–6, the medium was changed to DMEM/F12-based medium supplemented with ITS and 3 µM CHIR99021, 20 ng ml−1 FGF2 (Miltenyi Biotec), 0.5 µM LDN193189. On days 7–8, the medium was changed to DMEM/F12-based medium supplemented with 20 ng ml−1 FGF2, 0.5 µM LDN193189, 2 ng ml−1 IGF1 (Peprotech). On days 9–30, the medium was changed to DMEM/F12-based medium supplemented with 15% knockout serum replacement (Gibco, ThermoFisher Scientific), 1% penicillin and streptomycin, 0.05 mg ml−1 BSA (Sigma-Aldrich), 2 ng ml−1 IGF1.
Lung alveolar type 2 cell differentiation
Induced pluripotent stem cells were seeded onto flasks coated with Matrigel at a density of 0.5–1 × 104 cells per cm2 in primed hiPS cell medium (KSR/FGF2). After 48 h, the medium was changed daily with RPMI-based medium with B27 supplement (Gibco, ThermoFisher Scientific), 100 ng ml−1 activin A (Peprotech), 1 µM CHIR99021, 1% penicillin and streptomycin for 3 days. On days 4–8, the medium was changed daily with DMEM/F12-based medium with N2 (Gibco, ThermoFisher Scientific) and B27 supplements, 0.05 mg ml−1 ascorbic acid (Sigma-Aldrich), 0.4 mM monothioglycerol (Sigma-Aldrich), 2 µM dorsomorphin (Peprotech), 10 µM SB-431542 (Miltenyi Biotec), 1% penicillin and streptomycin. On days 9–12, the medium was changed daily with DMEM/F12-based medium with B27 supplement, 0.05 mg ml−1 ascorbic acid, 0.4 mM monothioglycerol, 20 ng ml−1 BMP4 (Peprotech), 0.5 µM all-trans retinoic acid (Sigma-Aldrich), 3 µM CHIR99021, 1% penicillin and streptomycin. On days 12–20, the medium was changed every other day with DMEM/F12-based medium with B27 supplement, 0.05 mg ml−1 ascorbic acid, 0.4 mM monothioglycerol, 10 ng ml−1 FGF10 (Stemcell Technologies), 10 ng ml−1 FGF7 (Peprotech), 3 µM CHIR99021, 50 nM dexamethasone (Sigma-Aldrich), 0.1 mM 8-bromoadenosine 3′,5′-cyclic monophosphate (Sigma-Aldrich), 0.1 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), 1% penicillin and streptomycin.
Flow cytometry
To obtain a single-cell suspension for flow cytometric analysis or sorting experiments, cells were collected using TrypLE express (Life Technologies) and resuspended in labelling mix (PBS, 2% FBS, 10 µM ROCK inhibitor Y-27632). Reprogramming intermediates and mature hiPS cells were labelled in a stepwise manner for cell surface markers. Step 1: F11R (mouse IgG antibody; 1:150), SSEA3-PE (rat IgM antibody; 1:10, BD Biosciences); step 2: Alexa Fluor 647 goat anti-mouse IgG (1:2,000, ThermoFisher), PE anti-rat IgM (1:200 eBioscience); step 3: CD13-PE-Cy7 (1:400, BD Biosciences), BV421-EpCAM (1:100, BD), TRA-1-60-BUV395 (1:100, BD Biosciences). Cells were incubated for 10 min on ice and then washed with PBS and resuspended in FACS buffer (PBS, 2% FBS, 10 µM Y-27632 and PI (1 in 500)). Prior to sorting, cells were passed through a 35-μm nylon filter. Sorted cells were collected for replating or downstream analyses. For differentiation experiments, cultures were dissociated using Accutase (Stemcell Technologies) and pelleted at 400g for 5 min. For neural differentiation experiments, cells were then resuspended in APC CD57 antibody (322314; Biolegend) and BUV395 CD56 antibody (563554; BD Biosciences); for muscle differentiation experiments, cells were resuspended in PE-Cy7 CD146 antibody (562135; BD Biosciences), BUV395 CD56 antibody (563554; BD Biosciences); for lung differentiation experiments, cells were resuspended in BV421 CD47 antibody (323116; Biolegend) and Brilliant Violet 421 CD326 antibody (324220; Biolegend); for NSC differentiation experiments, cells were labelled with BUV395 CD56 (NCAM) antibody and Alexa647 FAP antibody (FAB3715R; R&D Systems). Cells were resuspended in 2% fetal bovine serum (FBS; Gibco, ThermoFisher Scientific) and PBS (Gibco, ThermoFisher Scientific) and incubated for 15 min at 4 °C. The cell suspension was washed with PBS and pelleted at 400g for 5 min for analysis. Viability of cells was determined using propidium iodide solution (P4864; Sigma-Aldrich). Samples were analysed using an LSR IIb analyser (BD Biosciences) or a FACSAria II cell sorter (BD Biosciences) using BD FACSDiva software (BD Biosciences).
Immunostaining
Cells were fixed in 4% PFA (Sigma), permeabilized with 0.5% Triton X-100 (Sigma) in DPBS (ThermoFisher), and blocked with 5% goat serum (ThermoFisher). All antibodies used in this study are detailed in Supplementary Table 9 (for example, primary antibodies used were rabbit anti-NANOG polyclonal (1:100, Abcam) and mouse anti-TRA-1-60 IgM (1:300, BD Biosciences)). Primary antibody incubation was conducted overnight at 4 °C on shakers followed by incubation with secondary antibodies (1:400) for 1 h. After labelling, cells were stained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) (1:1,000, ThermoFisher) for 30 min. Images were taken using an IX71 inverted fluorescent microscope (Olympus). The following markers were assessed for respective differentiation assays: SOX17 and FOXA2 for endoderm progenitor differentiation experiments; SOX1 and PAX6 for neural differentiation experiments; PAX3 and PAX7 for skeletal muscle differentiation experiments; GATA6 and TTF1 for lung differentiation experiments.
Quantitative PCR with reverse transcription
RNA was extracted from cells using RNeasy micro kit (Qiagen) or RNeasy mini kit (Qiagen) and QIAcube (Qiagen) according to the manufacturer’s instructions. Reverse transcription was then performed using QuantiTect reverse transcription kit (Qiagen). Real-time PCR reactions were set up in duplicate using QuantiFast SYBR Green PCR Kit (Qiagen) and then carried out on the 7500 Real-Time PCR system (ThermoFisher) using LightCycler 480 software. The GAPDH gene was used to calculate the relative expression of each assessed gene. Information regarding the PCR primers used in this study is available in Supplementary Table 9.
 1 New Concepts in Science*
1 New Concepts in Science*